Abstract
Indolent splenic B-cell lymphomas are rare diseases (incidence <1:100,000) that include splenic marginal zone lymphoma (SMZL), hairy cell leukemia (cHCL), hairy cell leukaemia variant (HCLv), and splenic diffuse red pulp small B-cell lymphoma (SDRPL). These splenic lymphomas can have overlapping clinical presentations with only subtle differences in their morphology and phenotypes. However, there are considerable differences in prognosis, response to treatment, and outcomes. Better understanding of the tumor microenvironment (TME) is essential to design expedient and accurate diagnostic methods and precision drug treatments for each type of splenic B-cell lymphoma. We hypothesized each splenic lymphoma would have a distinct TME with some overlap for the red pulp lymphomas (cHCL, HCLv, and SDRPL).
Human specimen collection and usage was approved by the Institutional Research Subjects Review Board. We studied cryo-preserved bulk splenic tumor suspension cell isolates from fresh (<4 hour) splenectomy tissue and intact formalin-fixed paraffin embedded (FFPE) tissue sections from 17 splenic lymphoma patients and 4 trauma patients (controls). FFPE tissue sections were studied by immunohistochemical (IHC) staining and splenic suspension cells analyzed by high-parameter fluorescence flow cytometry with a 29-color myeloid panel using a Cytek Aurora spectral flow cytometer.
Initial IHC characterization of the splenic TME illustrated a markedly different distribution of intratumoral T cells (CD3) and macrophages (Mθ) (CD163). In cHCL, residual T cell zones appear retained, albeit diminished, while HCLv and SDRPL have markedly reduced T cells throughout with non-distinct zonation, and SMZL has prominent peritumoral collections of T cells at the interface of the neoplastic white pulp. Tissue architecture, as evidenced by splenic macrophage distribution, was markedly different. cHCL and HCLv showed diffuse but distorted networks of interdigitating macrophages, while SDRPL retained a nearly normal sinusoidal distribution of abundant macrophages, and SMZL showed total exclusion of CD163-positive Mθ from areas of confluent neoplastic white pulp.
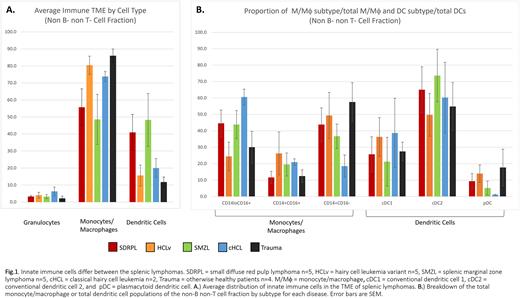
Analysis of splenic suspension cells showed an average of 72% B cells in the lymphoma specimens, twice that of trauma spleens. T cells made up the greatest fraction of non-B cells (~63%) for both the diseased and control samples. We analyzed the non-T and non-B cells to examine the innate immune cells. Granulocytes comprised on average 4% with no difference between the splenic lymphomas or trauma (Fig.1A). Trauma spleen had 86% macrophage/monocytes (M/Mθ)s, with similar values for HCLv (80%) and cHCL (74%). The percentage of M/Mθ was lower in SDRPL (55%) and SMZL (48%). We then analyzed the percentage of M/Mθ subtypes. As shown in (Fig.1B), CD14+CD16- was the major subtype for trauma (58%) and HCLv (50%). Conversely for cHCL, CD14loCD16+ was predominant (60%) subtype. SDRPL and SMZL shared similar subtype profiles with equal proportions of CD14+CD16- and CD14loCD16+ (40%). We then measured the dendritic cells of the innate immune population. SDRPL (41%) and SMZL (48%) had higher percentages than trauma (12%), HCLv (16%), or cHCL (20%). Conventional dendritic cell 2 (cDC2) was the predominant subtype comprising 50-74% of the DCs. Average conventional dendritic cell 1 (cDC1) and plasmacytoid dendritic cells (pDCs) were 30% and 9%, respectively. pDC was lower in cHCL than the other lymphomas (1%).
Our data show a similar M/Mθ component of the innate immune TME in the red pulp lymphoma SDRPL and white pulp lymphoma SMZL. SDRPL and SMZL also had higher proportions of dendritic cells compared to cHCL, HCLv, or trauma spleens. cDC2 was the predominant DC type in the spleen regardless of disease state. We are currently analyzing flow cytometry panels to access natural killer cell distribution and T cell functionality. We aim to better understand immune cell interactions in the TME and discover variations between the splenic B-cell lymphomas, resulting in improved diagnosis and therapy.
Disclosures
Peterson:Acerta/AstraZeneca: Research Funding; TG Therapeutics: Research Funding. Mosmann:Acerta/AstraZeneca: Research Funding; TG Therapeutics: Research Funding. Evans:Acerta/AstraZeneca: Research Funding; TG Therapeutics: Research Funding. Chu:Pfizer: Current equity holder in publicly-traded company. Zent:AstraZeneca: Research Funding; TG Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal